ATHENS WELLNESS (DIVISION OF ATHENS LIFE SCIENCES) - Best Pharmaceutical Company in Kala Amb Sirmaur, Himachal Pradesh
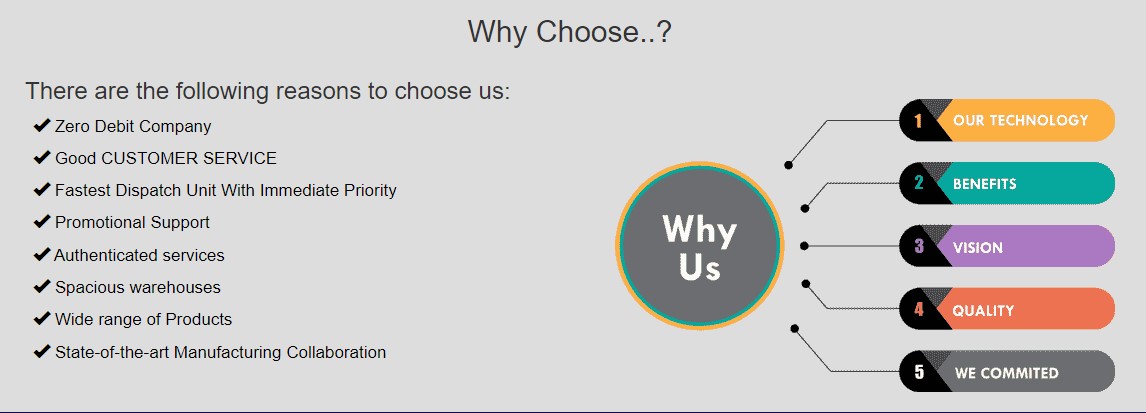
ATHENS LIFE SCIENCES is a WHO-GMP certified pharmaceutical manufacturing company based out of Kala Amb, Himachal Pradesh, India.
-The unit is manufacturing pharmaceutical products since 2004 (under the name of Athens Labs Ltd) and went through a constitution change for a name change in July 2017. (name changed to Athans Life Sciences) The Managing Director of the company is Mr. Anil Sharma who has 25+ years of experience in pharmaceutical industry and holds a diploma in Pharmacy.
The Director of the company is Mr. Gaurav Sardana who is loading Exports and Finance verticals.
The unit is managed by experienced Technical Staff for production & quality control along with a team of export Technicians
-The unit has approx. 5648 10 so meters plot with the total covered area about 3775.78 sq. Motors. The facilities include Raw and Packing material Warehouse, Finished Goods Warehouse and section for the manufacture of Tablot, Capsule, Liquid (General Category) and Tablet Capsule and Dry Syrup in bata-lactum Utilities include air-compressor, DG Set, RO Water System, Chiller. ETP Plant etc.
All the testing of Raw Intermediate and Finished Goods are done in our in house quality control lab arid Govt approved testing Laboratories.